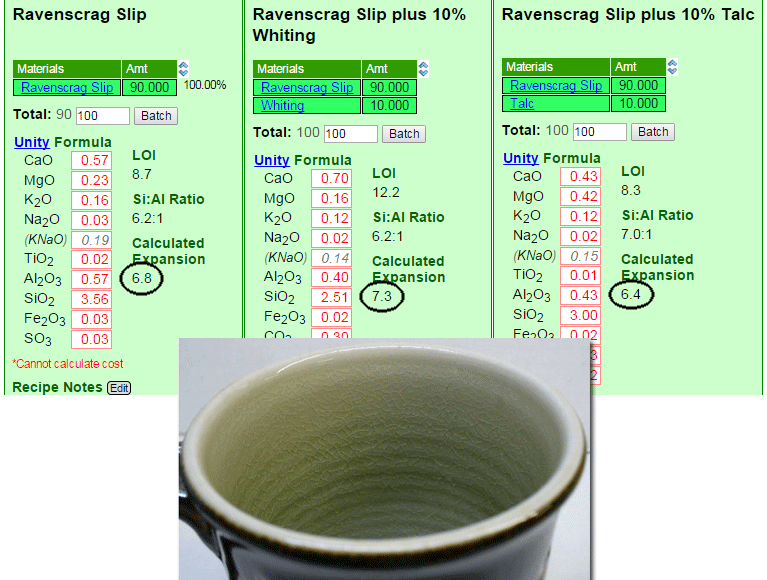
The unexpected reason for this crazing can be seen in the chemistry
The glaze is 10% insight-live.com/material/173">calcium carbonate added to Ravenscrag slip. Ravenscrag Slip does not craze when used by itself as a glaze at cone 10R on this body, so why would adding a relatively low expansion flux like CaO make it craze? This is an excellent example of the value of looking at the chemistry (the three are shown side-by-side in my account at Insight-live.com). The added CaO pushes the very-low-expansion Al2O3 and SiO2 down by 30% (in the unity formula), so the much higher expansion of all the others drives the COE of the whole way up. And talc? It contains SiO2 (so the SiO2 is not driven down nearly as much) and its MgO has a much lower expansion than CaO does.
Pages that reference this post in the Digitalfire Reference Library:
Talc, You cannot fix this crazing with a process or firing change, CaO is a strong flux but it can cause crazing, GR10-B Ravenscrag transparent glaze (with 10 talc), Calculated Thermal Expansion, Glaze Chemistry, Glaze Crazing

This post is one of thousands found in the Digitalfire Reference Database. Most are part of a timeline maintained by Tony Hansen. You can search that timeline on the home page of digitalfire.com.