High thermal expansion talc body cannot be COE-calculated
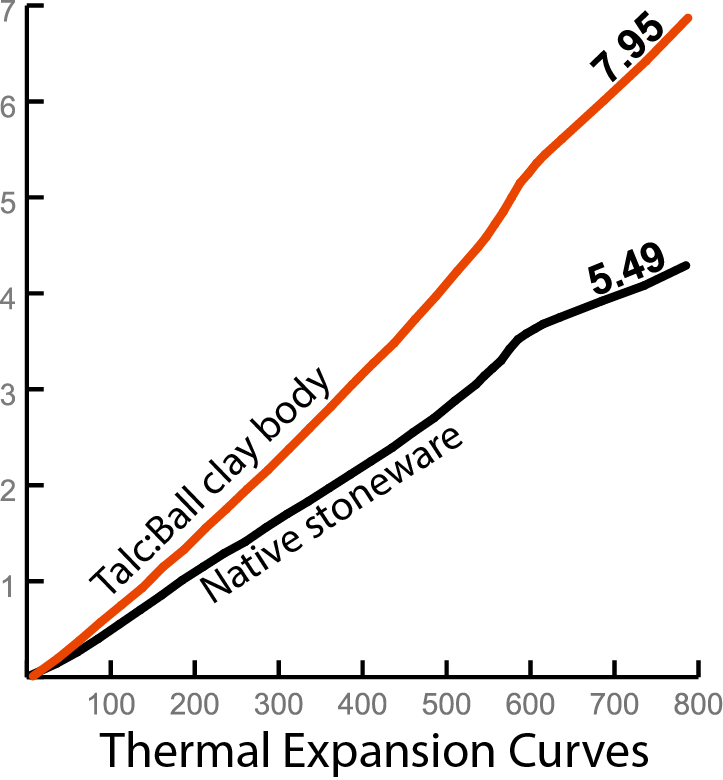
insight-live.com/material/1620">Talc is employed in low-fire bodies to raise their thermal expansion (to put the squeeze on glazes to prevent crazing). These dilatometer curves make it very clear just how effective that strategy is! The talc body was fired at cone 04 and the stoneware at cone 6. The former is porous and completely non-vitreous and the latter is semi-vitreous. This demonstrates something else interesting: The impracticality of calculating the thermal expansion of clay bodies based on their oxide chemistry. Talc sources MgO and low fire bodies containing it would calculate to a low thermal expansion. But the opposite happens. Why? Because these bodies are composed of mineral particles loosely sintered together. A few melt somewhat, some change their mineral form, many remain unchanged. The body's COE is the additive sum of the proportionate populations of all the particles. Good luck calculating that!
Pages that reference this post in the Digitalfire Reference Library:
Talc, G1916Q - Low Fire Highly-Expansion-Adjustable Transparent, Medium fire white bodies used at cone 04. Is that smart?, Co-efficient of Thermal Expansion, Glaze shivering, Calculated Thermal Expansion, Artware

This post is one of thousands found in the Digitalfire Reference Database. Most are part of a timeline maintained by Tony Hansen. You can search that timeline on the home page of digitalfire.com.