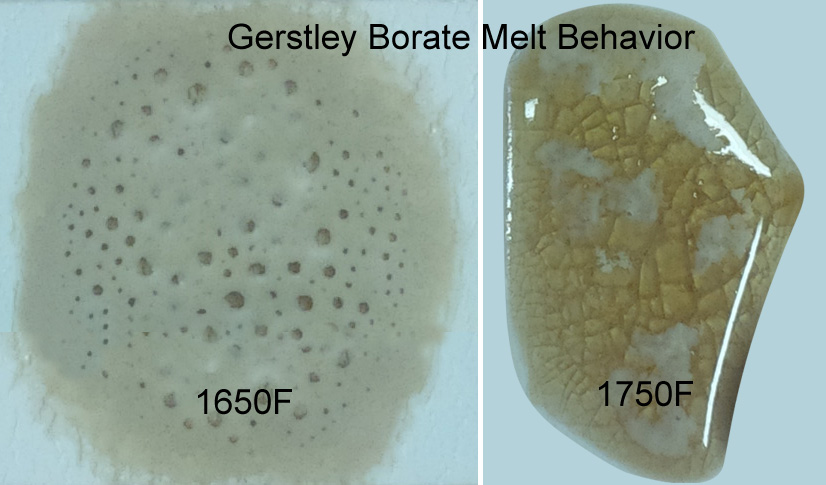
Why does Gerstley Borate melt in two stages? Because it is two minerals.
The insight-live.com/material/1660">ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile).
Pages that reference this post in the Digitalfire Reference Library:
Gerstley Borate, Ulexite, Colemanite, B2O3, Gerstley Borate vs Frit 3134 melt fluidity comparison, 954-Comparison of frit melts at 1750F

This post is one of thousands found in the Digitalfire Reference Database. Most are part of a timeline maintained by Tony Hansen. You can search that timeline on the home page of digitalfire.com.