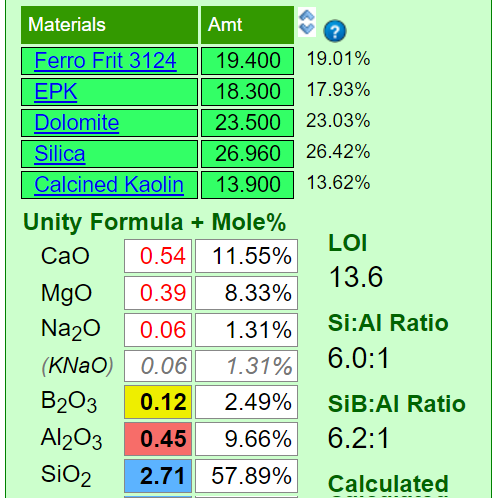
The Seger Unity Formula
Ceramic material powders can be insight-live.com/glossary/44">crystalline or amorphous (or a combination) and they can be natural or man-made and they can have very different particle physics. Ceramic materials are classic case studies of the sciences of solid state physics and solid state chemistry. Mineral sources of oxides impose their own melting patterns - when one is substituted for another, to supply an oxide, a different system with its own relative chemistry is entered. An extreme example of this is sourcing Al2O3 to a glaze using calcined alumina instead of kaolin. Although the chemical formula of the glaze may remain unchanged, the fired result would be completely different. This is because very little of the alumina would dissolve into the glaze melt. At the opposite extreme, a different frit could be used to supply a set of oxides (while maintaining the overall chemistry of the glaze) and the fired result would be much more chemically predictable. Why? Because they readily release their oxides to the melt.
Pages that reference this post in the Digitalfire Reference Library:
Frits vs. raw materials in glazes: It is not just about the chemistry, Oxide Formula, Unity Formula, Limit Formula, Mole%, Chemical Analysis, Glaze Chemistry

This post is one of thousands found in the Digitalfire Reference Database. Most are part of a timeline maintained by Tony Hansen. You can search that timeline on the home page of digitalfire.com.