CaO 0.65 K2O 0.15 Na2O 0.10 ZnO 0.10 B2O3 0.45 Al2O3 0.30 SiO2 3.00
In this example we will derive the recipe of materials needed to source the oxide formula of a zinc clear cone 6 glaze (of course the glaze also contains the fluxes CaO and KNaO, however since the vast majority of glazes also contain these, and, zinc is much less common, we call it a "zinc transparent").
Create Target Recipe
This method involves building the batch recipe in one panel while comparing the progress to the “target formula” in another panel (and doing material previews in a third panel).
Use the Add a Recipe button to create the target, title it as “Target Formula”.
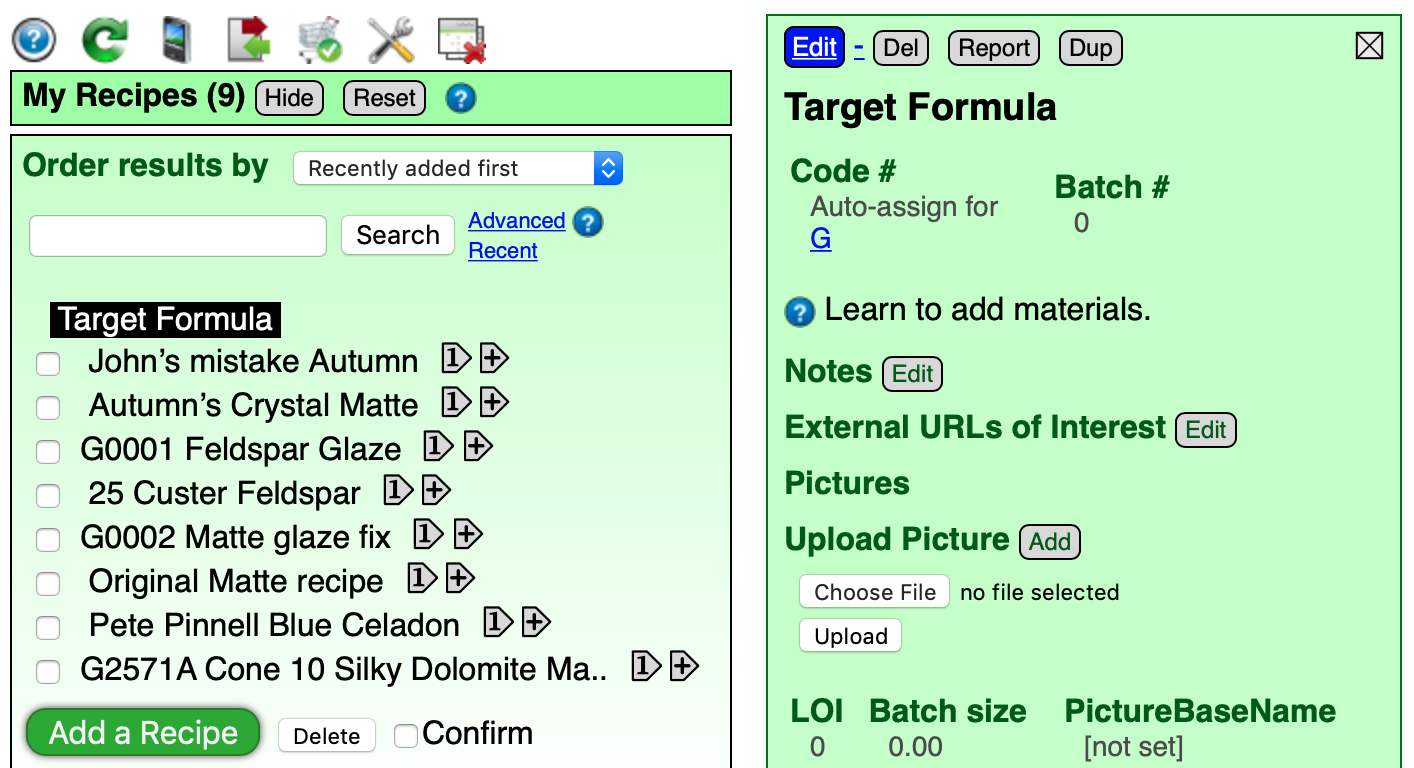
CaO 0.65 K2O 0.15 Na2O 0.10 ZnO 0.10 B2O3 0.45 Al2O3 0.30 SiO2 3.00
Here is the formula of the glaze. As text. Copy this to the clipboard and tap the “Import” button at the bottom of the green "Target Formula" panel.
The text importer page will appear.
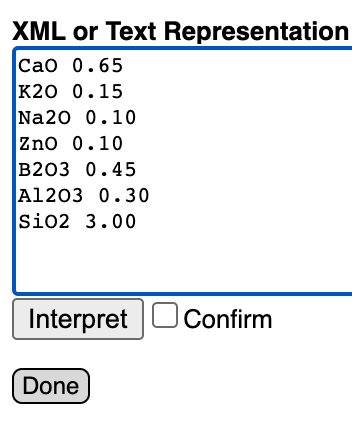
Select all the text already in the box (the XML representation of the recipe already in the panel) and paste the formula to replace it. At the bottom, check the Confirm box and then the Interpret button.
Here is what it looks like after import.
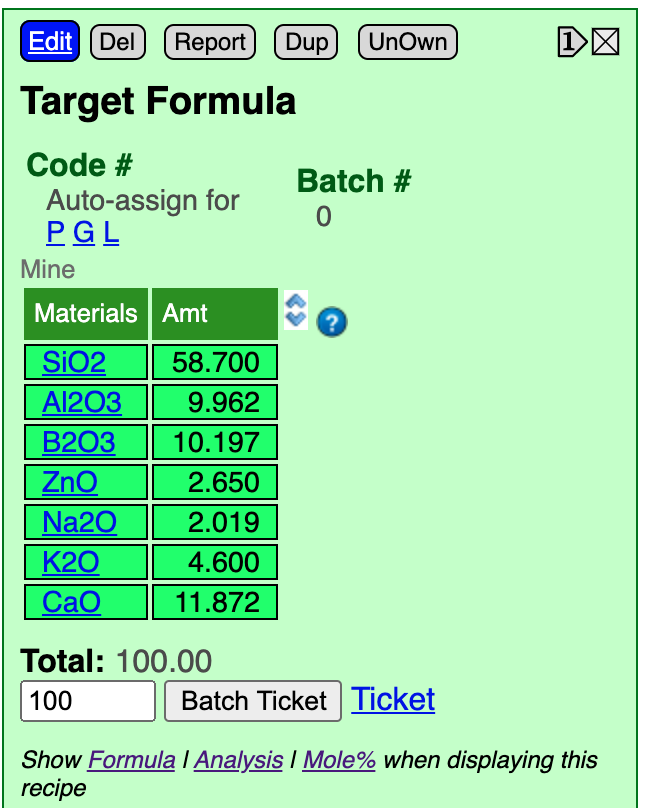
Insight knows we are entering chemistry instead of a recipe of materials because it recognized each line as an oxide name. It knows that we have entered a formula, rather than an analysis, because it totals less than 10. As a result, it has converted to a percentage analysis and puts that in as the recipe. This makes sense because a percentage analysis is technically a "recipe-by-weight of oxides". Although oxides are not available as pure materials, Insight is treating them as theoretic.
Tap the “Show Formula” link below the recipe. Insight will calculate the formula of our percentage analysis and show it below the recipe.
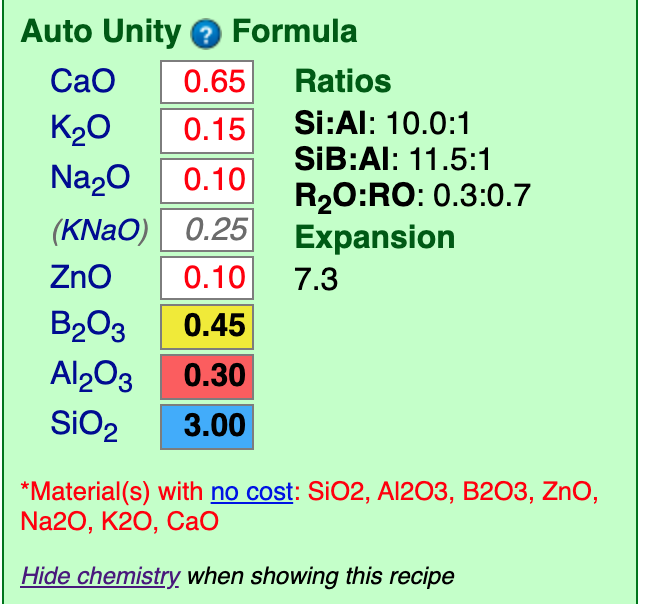
Notice that this is the same as the target formula we are working toward, verifying the the conversion-to-analysis worked correctly in the last step. It is also the same because the target formula we pasted was unified (the fluxes summed to one). It they had not, this would unify it and look different.
If you experience any issues with the import, just repeat it: Imported recipes replace the one in the panel.
Create Batch Recipe
Use the Add a Recipe button to create the batch recipe panel, title it as "Batch Recipe" and Save it. Then add six lines (a guess) using the Add materials button.
Then click the Done Editing button. Now it looks like this (do not click the Show Formula link below the recipe yet).
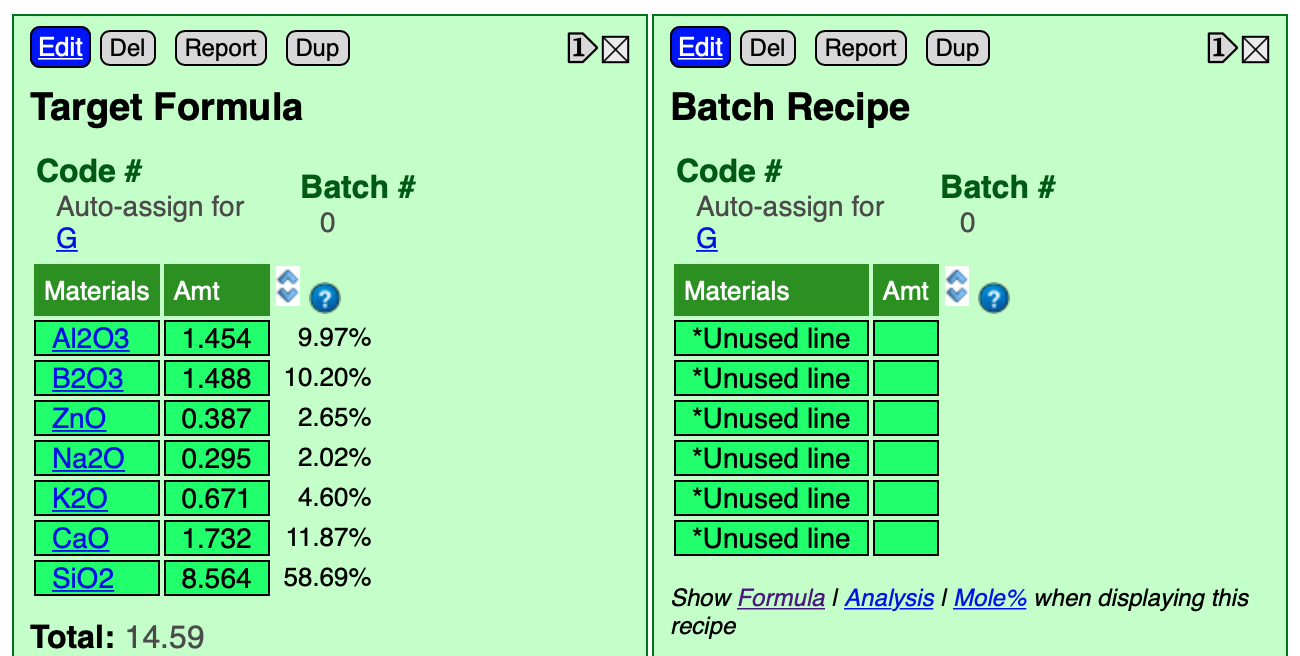
Supply B2O3 From Frit
Now we need to predict the materials to use. For this type of recipe for first material to consider is a frit to supply the B2O3 (Gerstley Borate could be used but it is too troublesome).
CaO 0.65 K2O 0.15 Na2O 0.10 ZnO 0.10 B2O3 0.45 Al2O3 0.30 SiO2 3.00
But which frit? Typically potters in North America have easy access to three common boron frits, Ferro 3134, 3124 and 3195. 3124 and 3195 are similar in that they can be a complete glaze with just the addition of 15% kaolin. This is because the frit chemistry already has significant Al2O3 so the entire burden of supplying it does not fall on the kaolin.
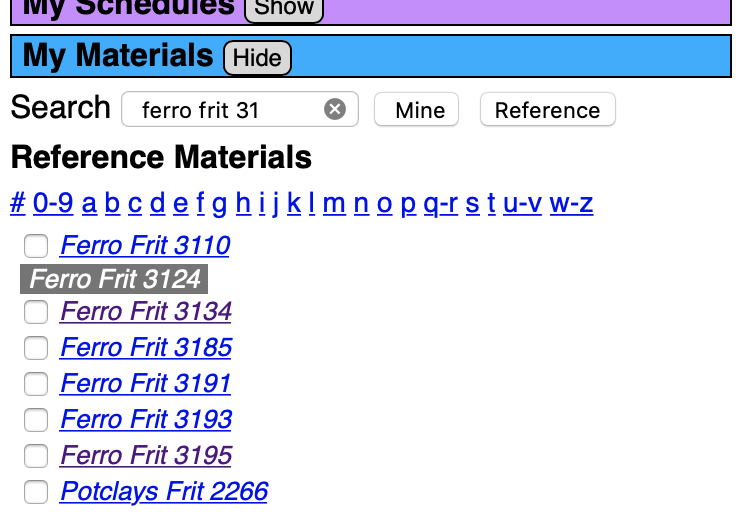
In the Materials Manager (the blue bar on the far left), search "ferro frit 31" (to find all three of the above). Click the Reference search button (to show the built-in materials). Click the individual frits and their chemistry info appears in a blue panel to the right of the target and batch recipe panels.
Here is what the whole screen looks like as we click materials on the left and they preview in a blue panel on the right. In the target formula we need 0.3 of Al2O3 (the one in the red box). That is a fairly low amount (common in glossy glazes), so we need to choose a boron frit with low Al2O3. Notice the chemistry of Frit 3134 here, it has only 2% Al2O3.
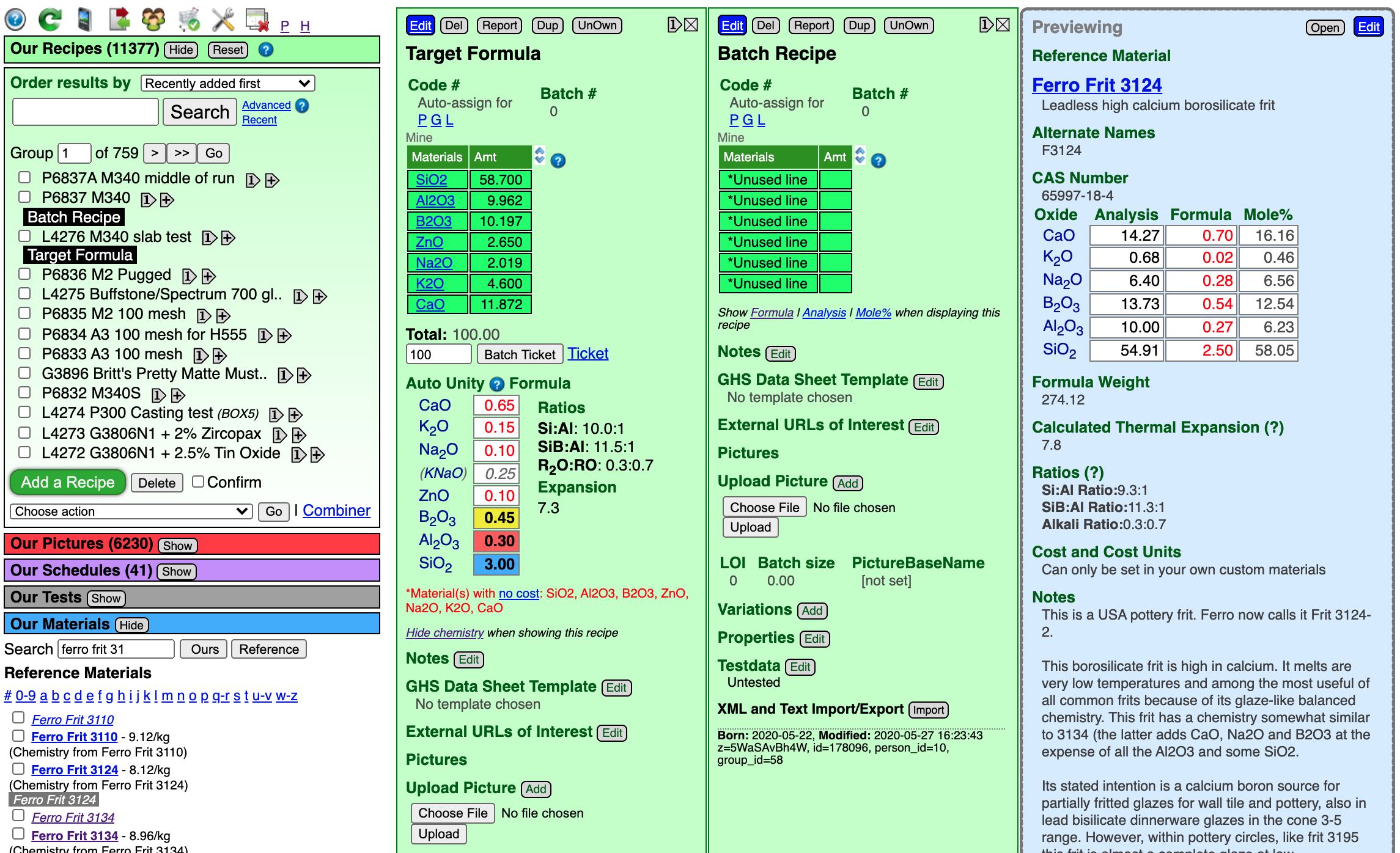
Click the blue Edit button at the top of the batch recipe panel. Enter the material "Ferro Frit 3134" (it has to be properly named to be recognized). We will guess an amount of 50. Click the Done Editing button. The click the Show Formula link (below the recipe) to show the chemistry for our batch recipe. Now we can see our target formula (on the lower left) and the materials we using to supply the oxides it on the right.
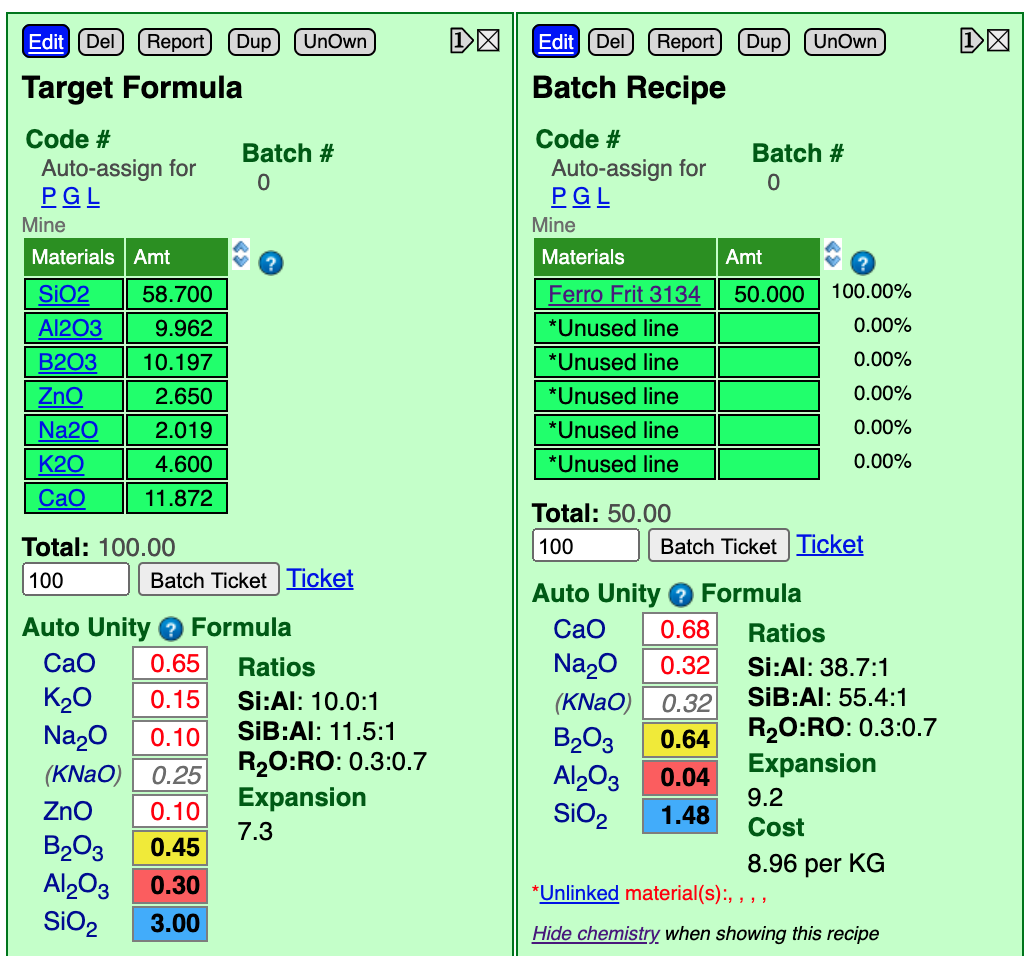
Notice that the frit supplies all the oxides needed except for one (ZnO). We are using the frit to source B2O3 (the yellow boxes). The guess of 50 sourced 0.64 whereas the target is 0.45. To match it we could repeat a cycle of editing the recipe, changing the amount, saving and checking, but let us look at a quicker way in the next step.
Using Calculation Mode
Calculation mode automates the nudging of the amounts of individual recipe lines up and down while watching the effect on the chemistry.
Enter calculation mode by clicking the  )
)
Click the ![]() and
and ![]() arrows to nudge material amounts by the value entered in the blank beside the double-arrows (each time you nudge the page will redisplay and recalculate the chemistry of the recipe).
arrows to nudge material amounts by the value entered in the blank beside the double-arrows (each time you nudge the page will redisplay and recalculate the chemistry of the recipe).
The default nudge amount is "1". Minimize the amount of nudges by changing this as appropriate. An effective strategy is to nudge in one direction until the desired oxide over or undershoots target, then divide the nudge amount in half and proceed in the opposite direction. Repeat until they match.
Terminate calculation mode by clicking 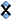 (it is also
is terminated by adding a new material to the recipe (and other actions).
(it is also
is terminated by adding a new material to the recipe (and other actions).
Since multiple recipes can be open side-by-side it is possible to adjust the chemistry of one while comparing it with another. To make it easier to compare specific oxide amounts, Insight-live colors the B2O3 (yellow), Al2O3 (red) and SiO2 (blue).
Matching the B2O3
To determine how much frit, to supply the needed 0.45 B2O3, we need to start by:
- Turn calculation mode on (as shown in the last step).
- Set the calculation type to Non-unity (by clicking the header above the formula until it cycles to non-unity).
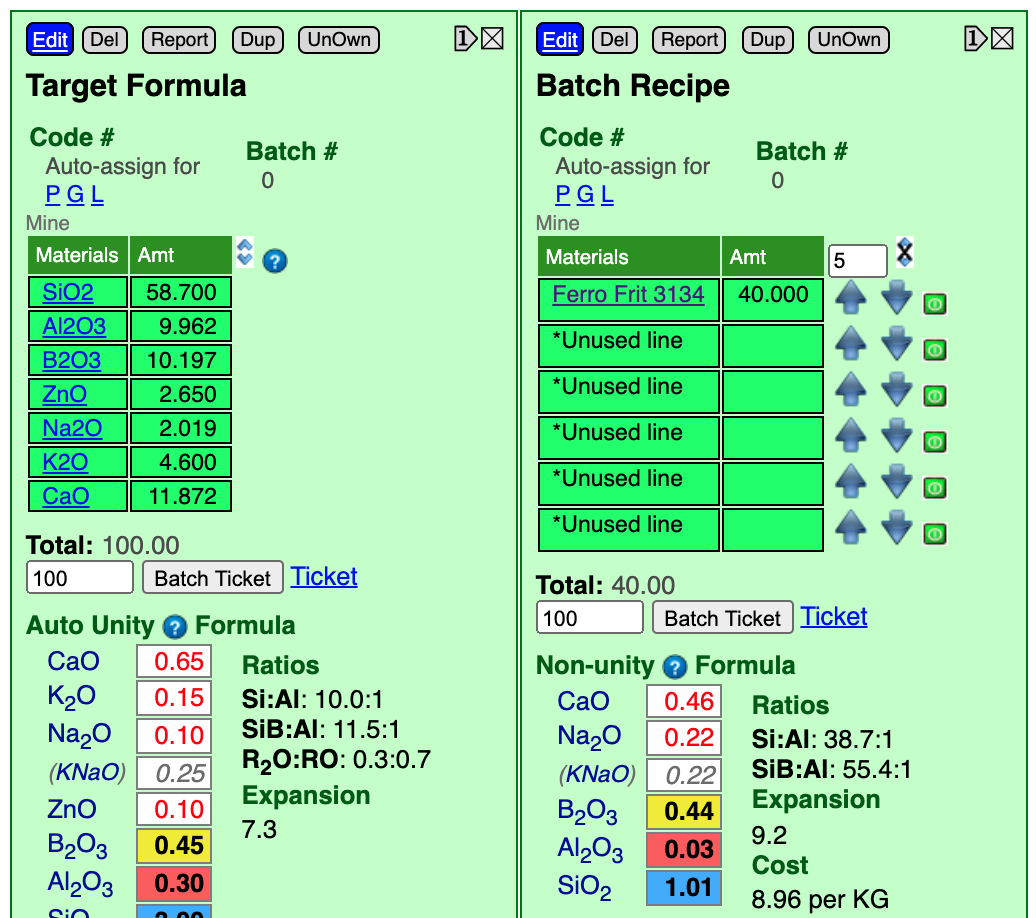
Notice that I have entered "5" into the increment box and have clicked the down-arrow beside the frit twice. That decremented it from "50" to "40". The B2O3 value (yellow box) has dropped to 0.44 (almost a match for the 0.45 target). This is stage 1 of the process, so that is accurate enough.
You might notice that, although the frit supplies many other oxides, none of them have overshot their targets. General purpose frits like this are designed that way. If any of them would have been substantially oversupplied we would have had to search for another boron frit.
What material should be added next? The one that supplies most oxides: Feldspar. Common feldspars supply both Na2O and K2O, and our target formula has them both. While it is possible to fiddle with a mix of feldspars to match these, since these oxides perform such similar functions in the chemistry it is common to group them together (referring to the pair as KNaO). So we will click the blue Edit button and add 20 parts of Custer Feldspar (a guess) to start the next step. And reduce the value in the increment box to 3 (when that is done you can click the feldspar name, if you are interested, and its chemistry will show to the right the way we did with the frit).
Supplying KNaO, ZnO, CaO
I have also clicked the down-arrow for the feldspar 4 times, that has reduced the KNaO to "0.26". This is, again, close enough for stage 1.
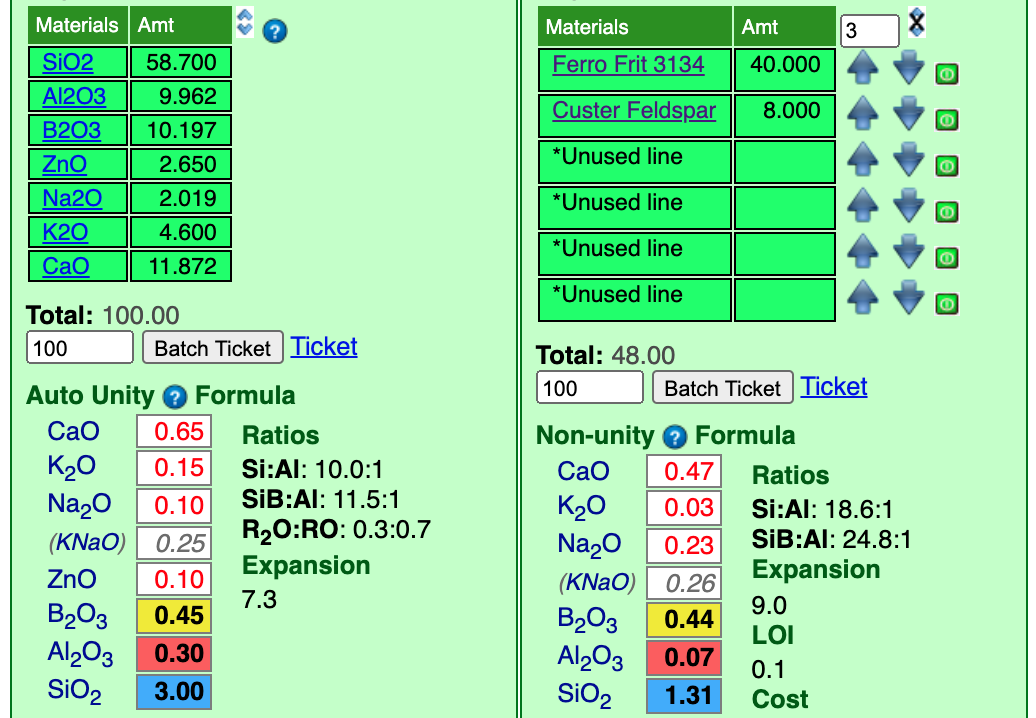
Next we will add the material Zinc Oxide. It is a concentrated material so we will start with just 2 parts. And an increment of 0.5.
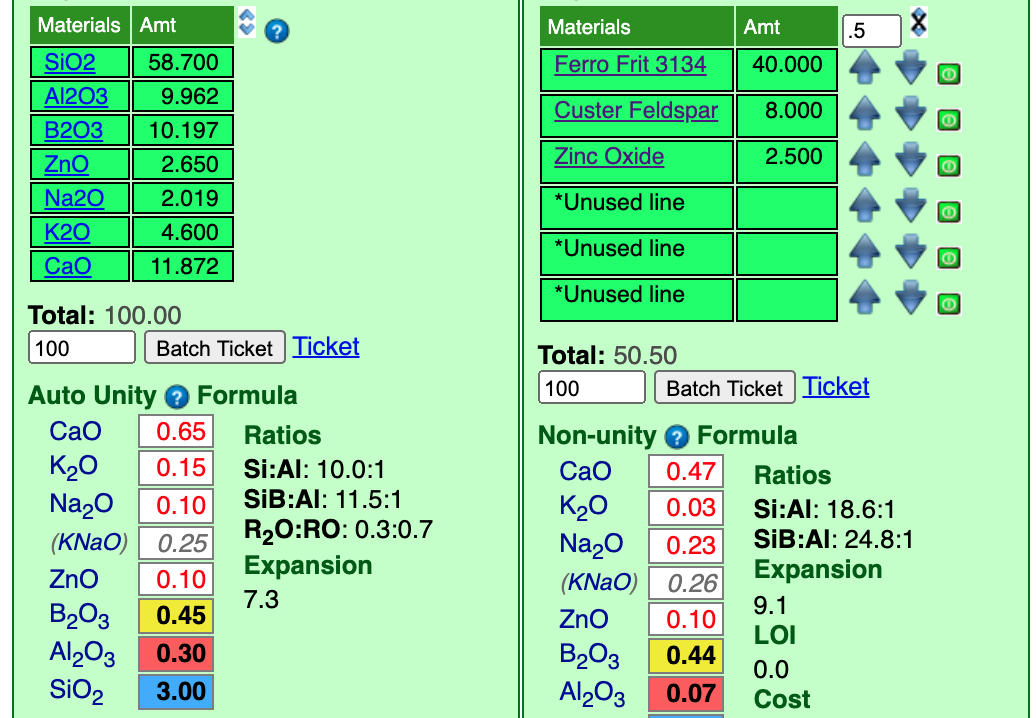
Notice it was only necessary to click the up-arrow beside zinc once to match the ZnO at "0.10".
Next, we will supply the CaO from calcium carbonate (whiting). The frit has already supplied most of the CaO needed so it is not going to take much feldspar. It is good when frits can supply a substantial part of the needed oxides since they melt better than raw materials. For whiting I will add 4 parts and set the incrementor at 1.
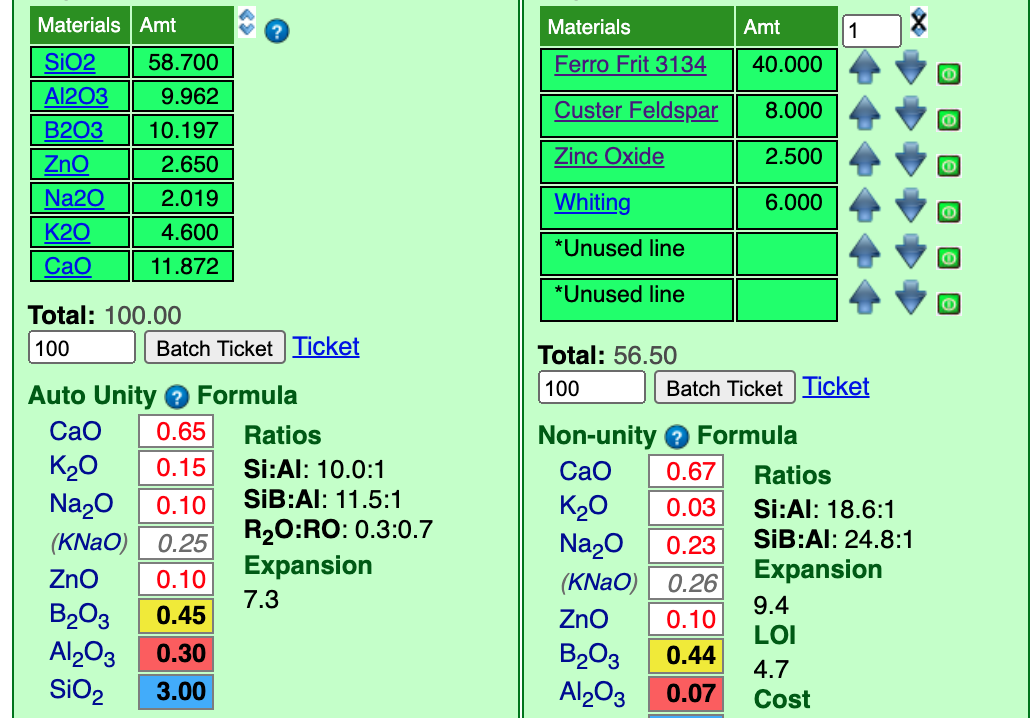
I have already clicked the up-arrow beside whiting twice and that took the CaO to "0.67". Again, that is close enough for now.
Supply Al2O3, SiO2
To supply Al2O3, I will add some EP Kaolin, and for SiO2 some silica. Quite a bit will be needed for both, so I will set them at "20" and the incrementor at "5".
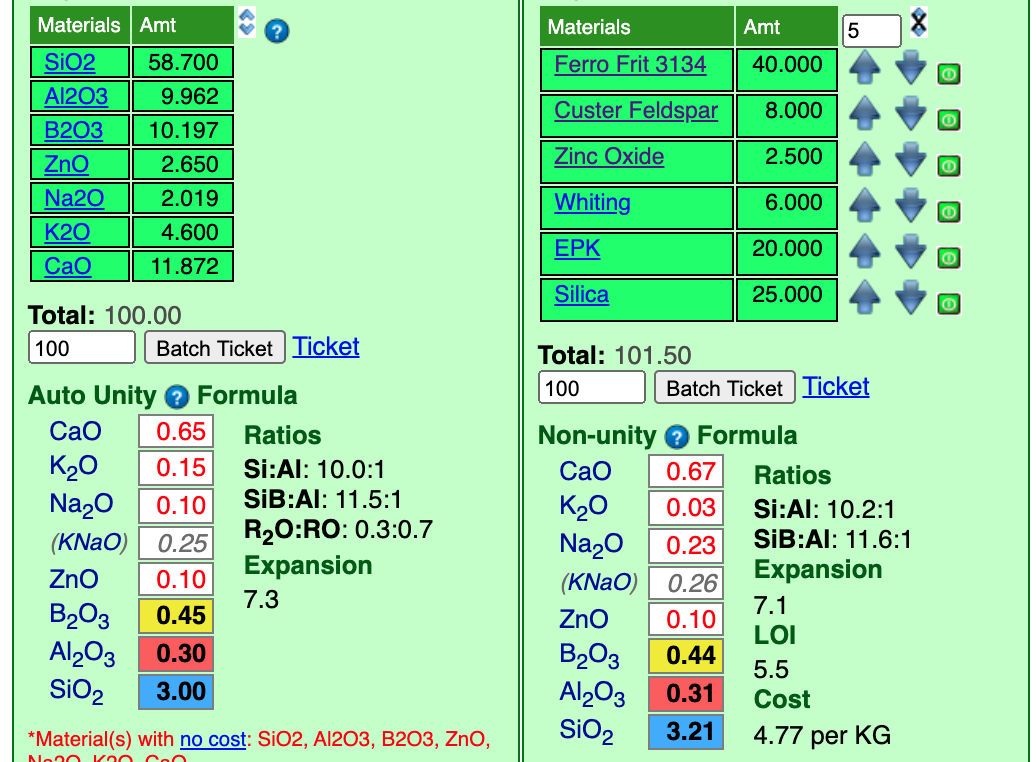
Notice that we lucked out on the Al2O3, it matches closely. The SiO2 was a little low so I have already up-arrowed the silica. That shot it well above the "3.0" target. So the incrementor is too big. I will reduce it to "1" and down-arrow the silica.
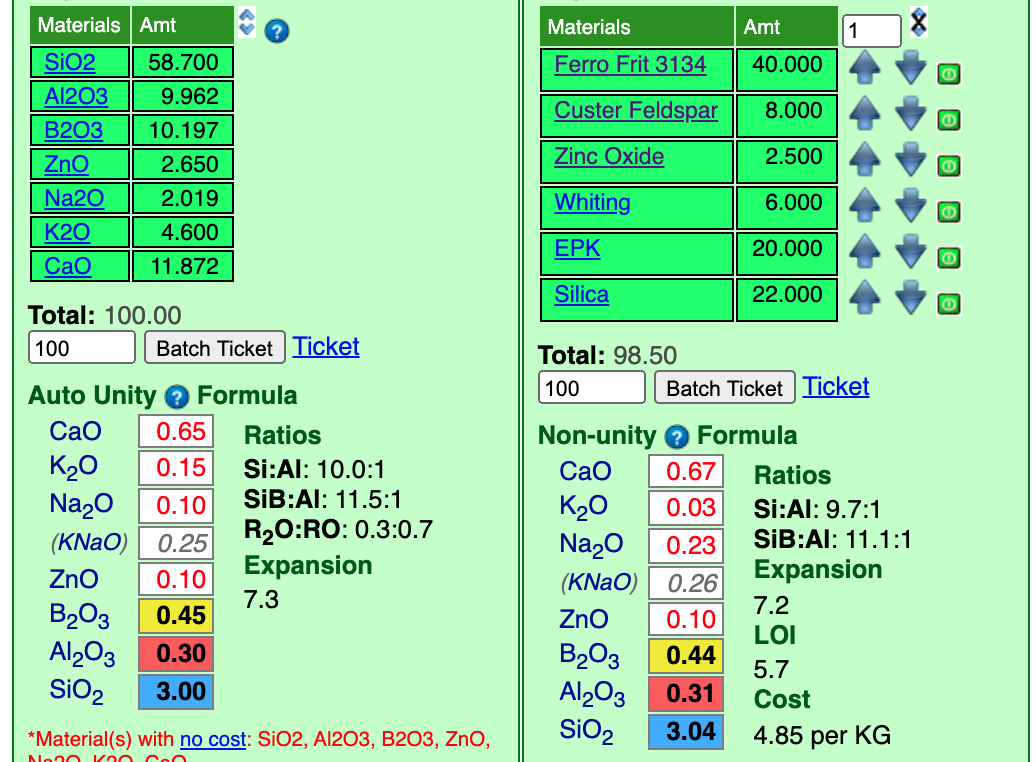
At "22.0" silica we have a fairly close match. Now our recipe totals 98.5. That was lucky! Last thing to do before we start stage 2 is change the unity setting from Non-unity to Auto Unity (that only requires one click).
Stage 2: Rationalization
Here is the recipe with its unity formula.
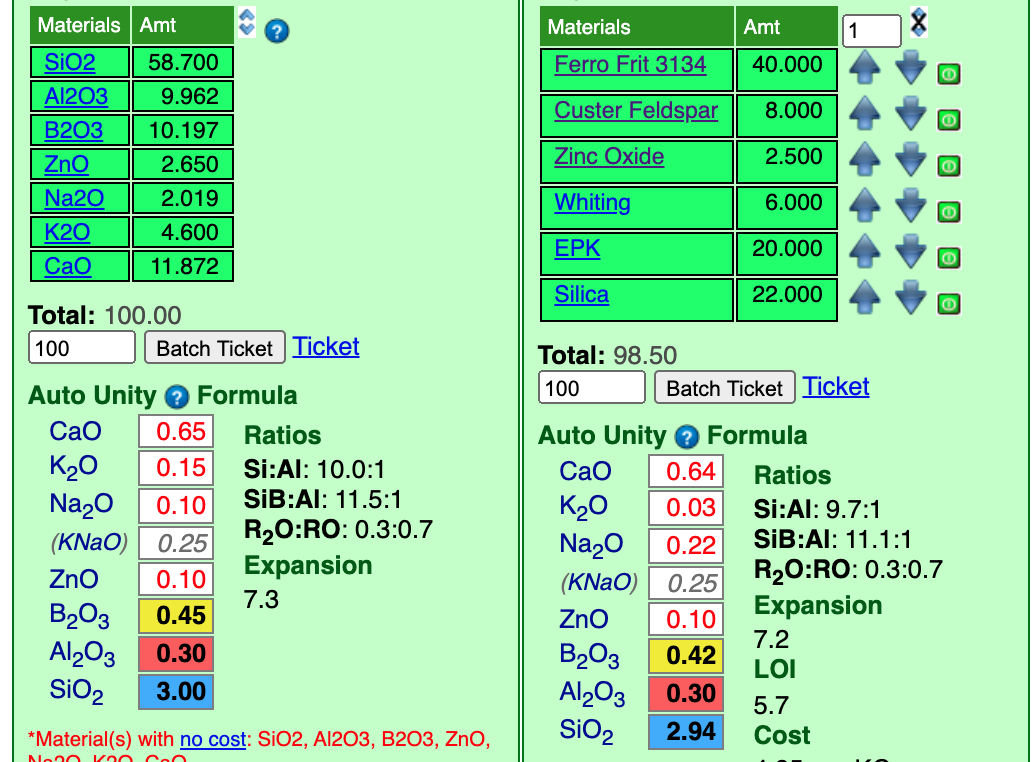
An Auto Unity setting means that Insight intelligently decides whether to set RO or R2O3 unity (in effect, we thus have RO unity). In stage 1 we were content with approximate matches for the oxide, the change to unity has changed the numbers a little. But they are close enough that we could decide that this recipe is OK. But before we tune it there is something else to consider.
The suspend, apply and harden well, glazes need enough kaolin (typically 15%). But if kaolin is too high (e.g. 25%) drying shrinkage is excessive and cracks develop (which lead to crawling during firing). This has "20" kaolin, so it should be good. If it had been 5-10, then it would have been necessary to use a low-alumina sodium frit (e.g. Ferro 3110) instead of feldspar to source the KNaO (enabling sourcing more Al2O3 from kaolin). If the kaolin was "30" then we could have incorporated some frit 3124 (to supply more Al2O3, thus requiring less kaolin). This process that we do at the beginning of stage 2 is called "rationalization". We have achieved the chemistry, but is the recipe sourcing that chemistry desirable? A frequent rationalized issue is cost. It could be that you might determine that the cost saving of using Gerstley Borate to source B2O3 would be worth the extra hassles that it would bring to the process (and inconsistencies in firing).
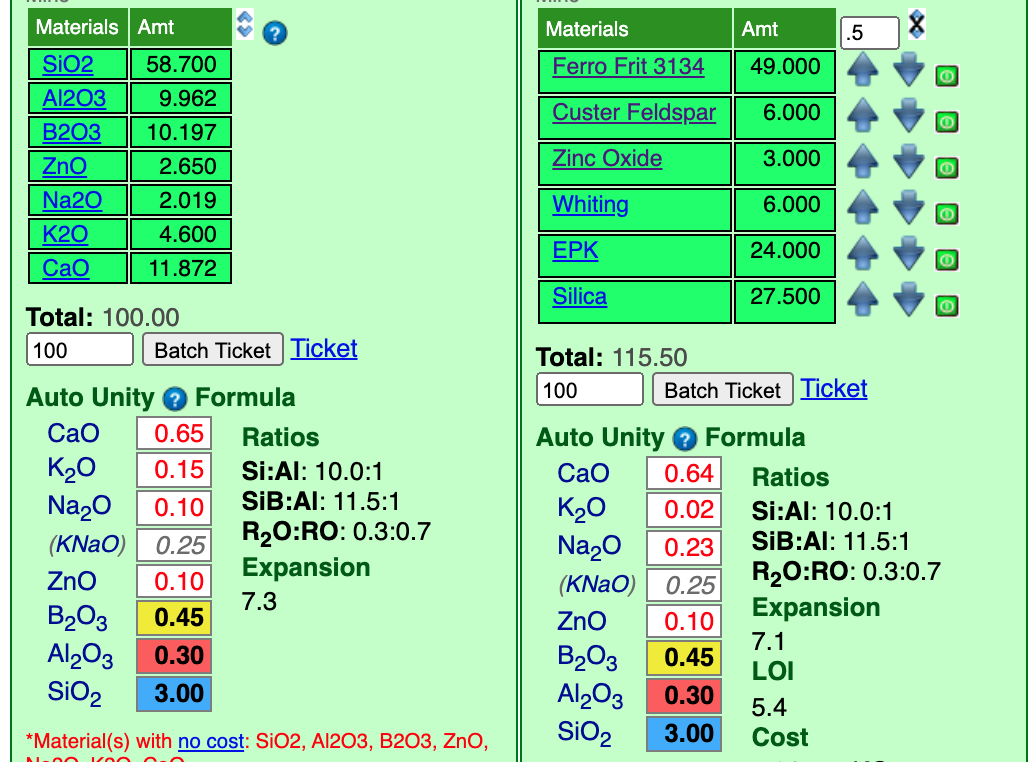
Two improve the match I upped the frit considerably (to match the B2O3). Because this is a unity formula, all the other numbers changed as I did that. Thus I had to tweak all the other materials (except the whiting). I made the changes in the same order as I added the materials. We now have a recipe that totals "115.5" so to finish we just need to retotal that to 100.
ReTotal the Recipe
Coming soon.