Project Name
Panama Cone 6 Ultra-Glossy Transparent Series
Project Codenumber
UnAssigned
Notes
After testing a number of cone 6 fluid melt, clear base glazes I found this one to have a number of properties I needed or liked:
-It was the most transparent and bubble free.
-Although it's thermal expansion was not as low as I would like, it was much better than some others and has potential to be reduced.
-As a base recipe also hosts the copper blue color effect well.
-It does not contain lithia (and thus the troublesome materials that source it) and only has a little zinc.
But it was not acceptable as is because it contained almost no clay so did not suspend or work as a slurry. Yet this problem was potentially easy-to-fix (and was).
As a clear glaze this has proven to work well on many bodies, producing the most brilliant gloss finish we have seen. It does craze on some porcelains and it does have some precipitate issues (see G3806D for improvement). Many glaze effects can only be achieved in a fluid melt, so a base like this is quite valuable.
Panama Blue Cone 6
|
Code # G3806 |
Batch # -2 |
| Materials | Amt |
|---|---|
| Custer Feldspar | 44.100 |
| Silica | 15.800 |
| Whiting | 2.600 |
| Kaolin | 2.600 |
| Dolomite | 7.700 |
| Strontium Carbonate | 4.200 |
| Ferro Frit 3110 | 10.700 |
| Ferro Frit 3134 | 9.700 |
| Zinc Oxide | 2.600 |
| Additions | |
|---|---|
| Tin Oxide | 2.600 |
| Copper Carbonate | 1.750 |
| Bentonite | 2.000 |
Total:106.35
Auto Unity Formula
|
Si:Al: 10.7:1 7.7 (Molar:7.3) 7.3 Cost 0.03 per kg |
Notes
This is the original recipe. It fires to a highly fluid melt and produces a brilliantly glossy base glaze. But it has very poor suspending and application properties (because of the low clay content). In addition, the green is only dark if it is very thick, and of course, that is not practical since it would run off the ware and craze also.
I initially attempted to transplant the tin and copper into my standard clear glaze base: G2926B. However the brilliant gloss was lost and it was a mass of half-healed blisters. Obviously the CO2 gas created from the decomposing copper need to be in a glaze fluid enough to enable them to bubble through. At first I thought about ways to make my base more fluid. However since I was aware of 3 other fluid-melt bases (that used different flux profiles) it seemed that it would be wise to try the chrome and tin in them all.
It was obvious that this recipe could be easily improved to address the poor slurry properties (by reducing the feldspar and supply the lost Al2O3 from kaolin and Na2O from Frit 3110). The copper could also be increased to intensify the color. That put me on the lookout to assess obvious problems with the other glazes I tried, and I reformulated them as appropriate also (one was unusable without change!).
Pictures
Glazes goes bumpy, matte after base change

The original glaze, Panama Blue (upper right). This test changes the clear base recipe to G2926B to see if the gloss will be maintained. It is not!
Upper right: Original high flux base with 1.75 copper oxide.
Bottom left: Copper in 2926B less fluid clear base applied thick.
Top left: Copper in 2926B base applied more thinly.
Panama Blue using Copper Oxide/Carbonate
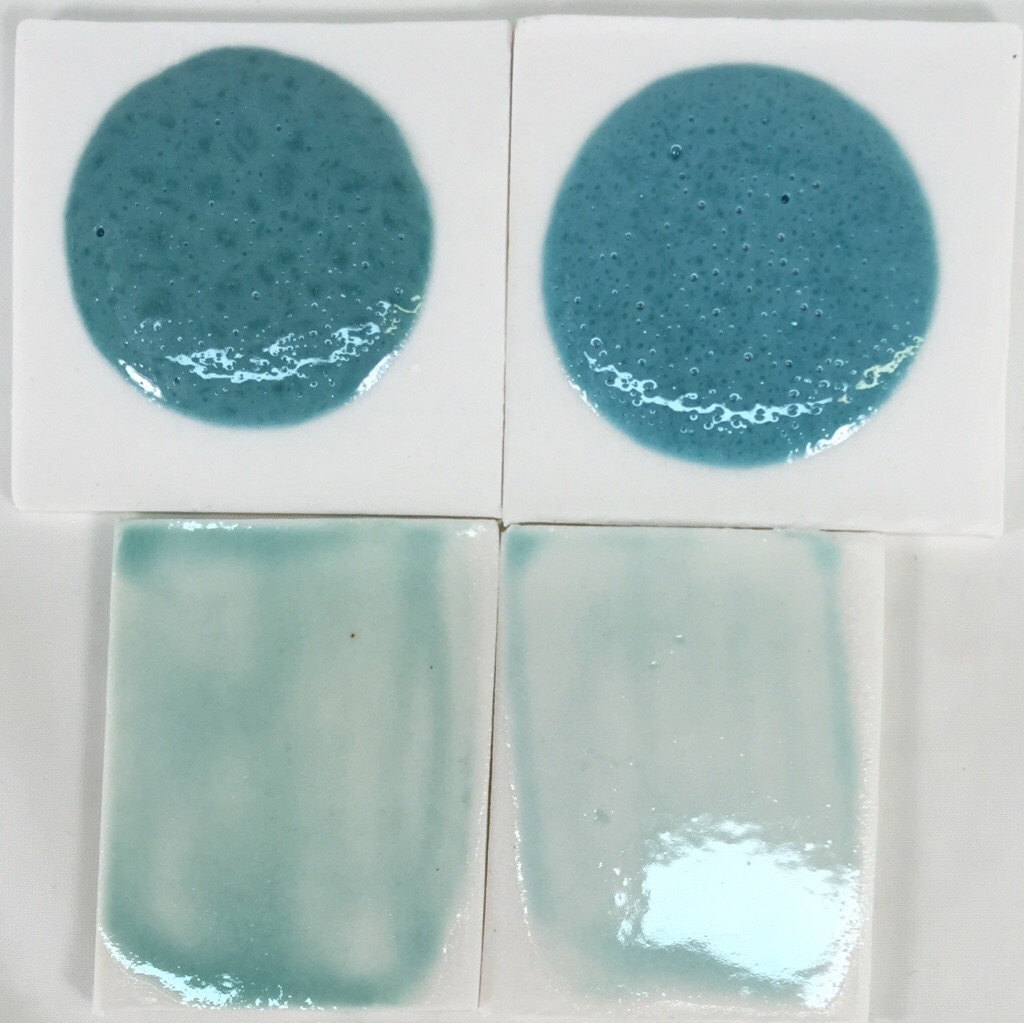
The top samples are 10 gram balls melted down onto porcelain tiles at cone 6. These demonstrate melt fluidity and susceptibility to bubbling.
Left: Original recipe using 1.75% copper carbonate.
Right: G3806B recipe using 1% copper oxide.
The copper oxide recipe is not bubbling any less even though copper oxide does not gas. Very strange. Extra bubbles must be coming from the kaolin.
The thin application on the tiles below demonstrates that the bubbles are not an issue. They also demonstrate that the glaze needs to be on fairly thick to get good color (or it needs more copper). The copper oxide one is not on as thick, otherwise it should be as dark.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama Blue Cone 6" id="74717" key="92aFZgCz" date="2017-01-07" codenum="G3806" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Custer Feldspar" amount="44.100" tolerance=""/> <recipeline material="Silica" amount="15.800" tolerance=""/> <recipeline material="Whiting" amount="2.600" tolerance=""/> <recipeline material="Kaolin" amount="2.600" tolerance=""/> <recipeline material="Dolomite" amount="7.700" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.200" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="10.700" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="9.700" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.600" tolerance=""/> <recipeline material="Tin Oxide" amount="2.600" added="true"/> <recipeline material="Copper Carbonate" amount="1.750" added="true"/> <recipeline material="Bentonite" amount="2.000" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2017-01-07 12:10:13
Panama Blue 2 - More clay, Copper Oxide
|
Code # G3806A |
Batch # -2 |
| Materials | Amt |
|---|---|
| Custer Feldspar | 11.500 |
| Silica | 20.000 |
| Whiting | 1.000 |
| Kaolin | 15.000 |
| Dolomite | 8.000 |
| Strontium Carbonate | 4.000 |
| Ferro Frit 3110 | 29.500 |
| Ferro Frit 3134 | 7.500 |
| Zinc Oxide | 2.500 |
| Additions | |
|---|---|
| Tin oxide | 2.600 |
| Copper Oxide | 1.000 |
Total:102.60
Auto Unity Formula
|
Si:Al: 10.8:1 7.5 (Molar:7.3) 7.4 Cost 0.30 per kg |
Notes
This adjustment reduces the feldspar in G3806 to supply more of the Al2O3 from kaolin (for better suspension). The KNaO lost is being supplied by more frit 3110. A little more SiO2 has been added.
This is an adjustment, an improvement to the original Panama Blue (G3806). This was part of a project to adjust it and compare it with other highly-melt-fluid copper blue bases proved to be a textbook example of the power of the glaze chemistry the way my insight-live.com account enables methodical documentation. It was possible to significantly alter the recipe to make it much better suspending while maintaining the chemistry as is (subsequent changes to the chemistry are likely to reduce the possibility of crazing). This recipe contains multiple sources of Na2O, Al2O3 and SiO2 making it possible to juggle them in many ways to effect a change in the oxide formula.
Compared to the original Panama Blue recipe, this variation sources more of the KNaO from Frit 3110 and much less from the feldspar. The resultant drop in Al2O3 (because of the lower feldspar) enables increasing the kaolin to supply it (that also removes the need for the bentonite).
I also compared this recipe with other fluid melt cone 6 clear bases, considering many factors and documenting as I went (much of it on Facebook). This one performed the best, especially in its slurry properties. The gloss is not quite a brilliant as Campana or Water blue, but not too far behind. As noted, there is one possible problem: The thermal expansion the Insight-live calculates is a little high so crazing could be an issue.
Mixing it with water: I used a ratio of 90 water to 100 powder, producing 1.4 Specific Gravity. It does not respond to vinegar as well as Epsom salts (likely because this SG is pretty low), I was patient in adding the Epsom Salts dry while mixing, it responded gradually and gelled beautifully. Do not try to use this with with too little water, it will not apply and dry nearly as evenly. At 1.4 specific gravity you might be surprised at how runny it is and how quickly it settles out, but the Epsom salts fixes that completely and transforms it into a glaze that applies like silk. There is a video on getting the thixotropy in a glaze right at http://digitalfire.com/videos.
Copper oxide is being used instead of carbonate, it has a low LOI, hopefully this will help avoid blistered if it goes on too thick.
Pictures
Panama Blue using Copper Oxide/Carbonate

The top samples are 10 gram balls melted down onto porcelain tiles at cone 6. These demonstrate melt fluidity and susceptibility to bubbling.
Left: Original recipe using 1.75% copper carbonate.
Right: G3806B recipe using 1% copper oxide.
The copper oxide recipe is not bubbling any less even though copper oxide does not gas. Very strange. Extra bubbles must be coming from the kaolin.
The thin application on the tiles below demonstrates that the bubbles are not an issue. They also demonstrate that the glaze needs to be on fairly thick to get good color (or it needs more copper). The copper oxide one is not on as thick, otherwise it should be as dark.
URLs
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama Blue 2 - More clay, Copper Oxide" id="74741" key="2fM7Q5Yt" date="2017-01-07" codenum="G3806A" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Custer Feldspar" amount="11.500" tolerance=""/> <recipeline material="Silica" amount="20.000" tolerance=""/> <recipeline material="Whiting" amount="1.000" tolerance=""/> <recipeline material="Kaolin" amount="15.000" tolerance=""/> <recipeline material="Dolomite" amount="8.000" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.000" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="29.500" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="7.500" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.500" tolerance=""/> <recipeline material="Tin oxide" amount="2.600" added="true"/> <recipeline material="Copper Oxide" amount="1.000" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2017-01-07 12:23:43
Panama Blue 3 - Copper Carbonate
3110, 3134, zinc, Sr
|
Code # G3806B |
Batch # -2 |
| Materials | Amt |
|---|---|
| Custer Feldspar | 11.500 |
| Silica | 20.000 |
| Whiting | 1.000 |
| Kaolin | 15.000 |
| Dolomite | 8.000 |
| Strontium Carbonate | 4.000 |
| Ferro Frit 3110 | 29.500 |
| Ferro Frit 3134 | 7.500 |
| Zinc Oxide | 2.500 |
| Additions | |
|---|---|
| Tin Oxide | 2.500 |
| Copper Carbonate | 2.000 |
Total:103.50
Auto Unity Formula
|
Si:Al: 10.8:1 7.5 (Molar:7.3) 8.1 Cost 0.01 per kg |
Notes
After a year of storage we found a considerable amount of hard dark precipitate lumps stuck to the bottom and walls of glaze bucket. Very hard and had to scrape off with fettling knife. This could be because of solubility of Frit 3110.
Pictures
Fluid cone 6 clear glazes

These are 10 gram glaze balls are fired down onto tiles to demonstrate melt fluidity and bubbling.
Left: L3808 GB clear from Shaun Mollonga (most fluid).
G3808A fritted recalculation of former (best surface).
G3813 Campana clear (most transparent).
G3806B Panama Blue base.
All of these survived 260F:Icewater test without crazing on M370, M390 and M340.
Campana Clear is the smoothest on M340, Panama is second best.
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents

The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
Compare four clear bases for copper blue

Has extenal picture also
The flow testers at the back and the melt-down-balls in from of them have 1% copper carbonate. The glazed samples in the front row have 2% copper carbonate. L3806B, an improvement on the Panama Blue recipe, has the best color and the best compromize of flow and bubble clearing ability.
2% Copper carbonate in two different cone 6 copper-blues

The top base glaze has just enough melt fluidity to produce a brilliant transparent (without colorant additions). However it does not have enough fluidity to pass the bubbles and heal over from the decomposition of this added copper carbonate! Why is the lower glaze passing the bubbles? How can it melt better yet have 65% less boron? How can it not be crazing when the COE calculates to 7.7 (vs. 6.4)? First, it has 40% less Al2O3 and SiO2 (which normally stiffen the melt). Second, it has higher flux content that is more diversified (it adds two new ones: SrO, ZnO). That zinc is a key to why it is melting so well and why it starts melting later (enabling unimpeded gas escape until then). It also benefits from the mixed-oxide-effect, the diversity itself improves the melt. And the crazing? The ZnO obviously pushes the COE down disproportionately to its percentage (although there is further to go because it is crazing somewhat).
Copper Blue G8306C using copper carbonate, oxide

Right is G3806C, an adjustment to drop the thermal expansion of B. It does this by trading some of the high-expansion KNaO for a mix of MgO, ZnO and SrO. These is an improvement but it still could craze over time on high-kaolin or low silica porcelains.
One more change: The one on the right uses 2% Copper Oxide instead of 2% Copper Carbonate (left). Both also add 2.5% tin oxide. Strangely the color is only slight darker (the oxide is a more concentrated form of copper than the carbonate).
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama Blue 3 - Copper Carbonate" keywords="3110, 3134, zinc, Sr" id="75240" key="fsdy2BQf" date="2017-01-07" codenum="G3806B" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Custer Feldspar" amount="11.500" tolerance=""/> <recipeline material="Silica" amount="20.000" tolerance=""/> <recipeline material="Whiting" amount="1.000" tolerance=""/> <recipeline material="Kaolin" amount="15.000" tolerance=""/> <recipeline material="Dolomite" amount="8.000" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.000" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="29.500" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="7.500" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.500" tolerance=""/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> <recipeline material="Copper Carbonate" amount="2.000" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2017-01-07 12:21:33
Panama Cone 6 Adjustment 2015
High fluid melt glaze for reactive effects and super gloss colors
|
Code # G3806C |
Batch # -2 |
| P | Materials | Amt | |
|---|---|---|---|
| Silica | 26.300 | 26.27% | |
| Kaolin | 19.700 | 19.68% | |
| Dolomite | 8.700 | 8.69% | |
| Strontium Carbonate | 4.400 | 4.40% | |
| Ferro Frit 3110 | 31.100 | 31.07% | |
| Ferro Frit 3134 | 6.600 | 6.59% | |
| Zinc Oxide | 3.300 | 3.30% |
| P | Additions | ||
|---|---|---|---|
| * | Copper Oxide | 2.000 | 2.00% |
| * | Tin Oxide | 2.500 | 2.50% |
Total:104.60
Auto Unity Formula
|
Si:Al: 11.1:1 7.3 (Molar:7.1) 7.9 |
Notes
This is work I did in 2015 (in 2019 a much bigger project developed this further).
The copper and tin produce the turquoise celadon effect.
This recipe is for a brilliant fluid-melt transparent base glaze, initially for copper blues and greens, but later for stains. "Fluid-melt" means it runs down off ware if applied too thickly, this is a key for achieving many visual effects.
Initiailly I compared a number of recipes I found on line and finally selected Panama Blue. I removed the colorants and made adjustments to improve slurry properties and lower the thermal expansion (it has serious crazing issues). Fluid-melts have a down side: Crazing is an issue (because the fluid melt requires more fluxes, these have higher thermal expansions).
Then I did three adjustments, each lowering the thermal expansion more than the last. While keeping the same brilliant visual appearance. The recipe ended up being quite different (two materials were eliminated from the recipe, their oxides supplied by the others). The chemistry of this one moves much of the KNaO to low-expansion MgO. This makes it melt a little less, but visually it is the same. Higher ZnO helps melting (since MgO is not nearly as powerful a flux as KNaO). I was even able to add extra SiO2. The calculated thermal expansion has gone from 7.7 down to 7.3.
This worked well on stonewares but still crazed on Plainsman P300 and M370 (but was OK on Polar Ice). Fluid melt glazes look best on porcelains so this was obviously a problem. So I continued development in pursuit of a fluid melt having a lower thermal expansion (see subsequent articles, recipes and posts).
Pictures
Copper Blue G8306C using copper carbonate, oxide

Right is G3806C, an adjustment to drop the thermal expansion of B. It does this by trading some of the high-expansion KNaO for a mix of MgO, ZnO and SrO. These is an improvement but it still could craze over time on high-kaolin or low silica porcelains.
One more change: The one on the right uses 2% Copper Oxide instead of 2% Copper Carbonate (left). Both also add 2.5% tin oxide. Strangely the color is only slight darker (the oxide is a more concentrated form of copper than the carbonate).
Plainsman P300, M370 with copper blue glaze cone 6

This is the G3906C base plus 2.5% tin oxide and 2% copper oxide. The green glaze does craze over time on these bodies, but the inside glaze is a liner than will not.
3806C vs. other cone 6 clear glazes on a dark stoneware

Each pair of mugs shows a numbered glaze vs. G3806C on the right. The body is a red burning cone 6 stoneware, Plainsman M390.
G2926B, 3806C vs. Amaco C11 Clear at cone 6

Bottom right is P300 with three coats of C11.
Bottom left: 10 gram ball of C11.
2926 B is top left, 3806C is top right.
G3806C Copper Blue on Polar Ice

Polar Ice is the easiest of Plainsman middle fire porcelains to fit a glaze to, although this glaze crazes on most other porcelains, it should stay craze free on this.
G3806C on a dark burning cone 6 stoneware

Plainsman M390. There is still some clouding, but it is better than other transparents we have used.
G3806D melt flow test

Left is G3806C with copper oxide 2%. Right is G3806D with copper carbonate 2%. The melt fluidity is identical. The blue color thus seems to depend on the carbonate (or a lower percentage of the oxide is needed).
Variations
1 - Midnight
Fire fast to 2100F (300-400F/hr), then 100F/hr to 2200F, then drop fast to 2000F and soak half hour, then cool at 100F/hr to 1400F.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama Cone 6 Adjustment 2015" keywords="High fluid melt glaze for reactive effects and super gloss colors" id="75786" key="BPSy4iMf" date="2024-07-24" codenum="G3806C" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="26.300" tolerance=""/> <recipeline material="Kaolin" amount="19.700" tolerance=""/> <recipeline material="Dolomite" amount="8.700" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.400" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="31.100" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="6.600" tolerance=""/> <recipeline material="Zinc Oxide" amount="3.300" tolerance=""/> <recipeline material="Copper Oxide" amount="2.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2024-07-24 17:59:11
